Human Congenital Heart and Limb Malformations
Research Focus
The most common human birth defects originate from faulty self-organization of mesodermal precursors during embryogenesis. The resulting congenital disorders of mesodermal derivatives, such as the heart and limbs, affect many pregnancies, children, and a growing number of adults with lifelong complications. Although the heart and limbs are very different organs, they share the underlying gene regulatory networks to a large extent. As a result, patients with limb malformations often have heart defects, but we do not understand the underlying mechanisms. Moreover, mesodermal developmental genes and processes also play an important role in the etiology of adult cardiovascular disease, the primary cause of death worldwide (32% of all deaths, WHO), due to a lack of treatment. Thus, insights into how disrupted gene regulatory networks lead to heart and limb malformation, and how they are linked to adult heart disease, will pave the way to the development of much-needed diagnostic and treatment strategies.
Main Objectives
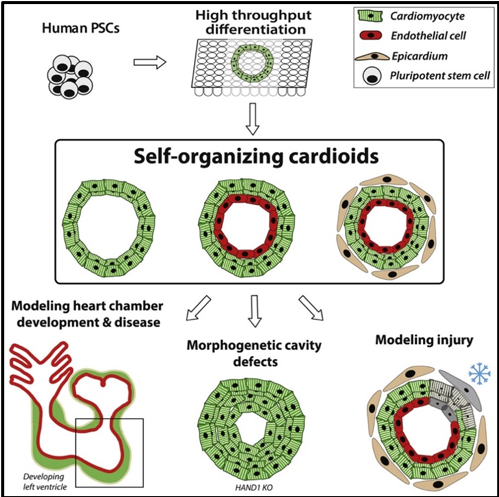
We aim to investigate how shared signaling and gene expression networks drive the self-organization of the heart and forelimbs, and how teratogens and mutations cause malformations in these organs. To achieve this, we utilize human pluripotent stem cell (hPSC) differentiation and the derivation of organoids, which have revolutionized human developmental biology and opened up new diagnostic and therapeutic avenues. Specifically, our lab has developed chamber-like cardiac organoids (multichambered cardioids) (Hofbauer et al. 2021, Schmidt et al. 2023) as well as human limb bud organoids (Röcklinger et al, in revision), which we can use to dissect the underlying molecular causes of congenital malformations.
References
- Hofbauer P, Jahnel SM, Papai N, et al. Cardioids reveal self-organizing principles of human cardiogenesis. Cell. 2021;184(12):3299-3317.e22. doi:10.1016/j.cell.2021.04.034
- Schmidt C, Deyett A, Ilmer T, et al. Multi-chamber cardioids unravel human heart development and cardiac defects. Cell. 2023;186(25):5587-5605.e27. doi:10.1016/j.cell.2023.10.030
Content of Research
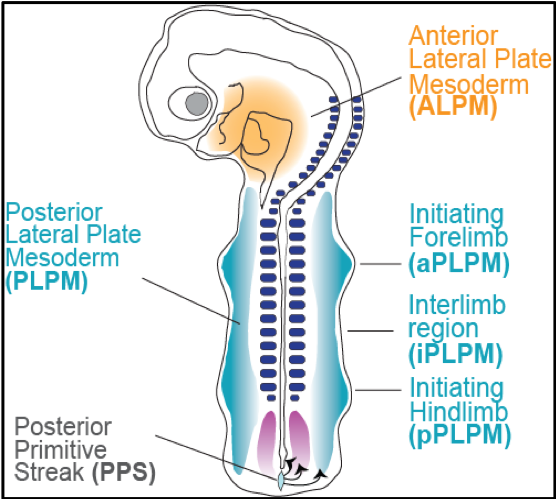
The crucial limb specification events in the developing embryo take place during Carnegie stages 10 and 11 (E22-E26). However, limited embryonic material is available from these stages, dictating the urgent need for a reliable in vitro organoid model. Previous work from our lab has established such a model in 2D and 3D, demonstrating formation of pre-limb bud structures (Röcklinger et al, in revision). We aim to leverage the human limb bud organoid model to study the effects of thalidomide and its derivatives, as well as other potential teratogenic drugs and materials, in forelimb bud formation. We will investigate these effects at the morphological level, as well as at the whole-proteome and single-cell RNA and proteomic levels. The results of the study will allow us to comprehend the molecular mechanism of teratogen-induced malformations in the forelimb. The potential connection of this mechanism to heart development will also be investigated in our established multi-chambered cardioid model. Such knowledge would be necessary for the design of improved drugs and/or materials to prevent teratogen-induced congenital defects in the limb and heart.
In a second ongoing lab project, we mediate the collaboration between the Mendjan lab at the Institute of Molecular Biotechnology (IMBA) and pre-clinical and clinical researchers at the MUW & Vienna General Hospital (AKH) to compare cardioids with patient-derived tissue. Our general aim is to develop the cardioid platform as close as possible to a newborn, child, and ultimately an adult heart. Our hypothesis is that organoids need to recapitulate development as faithfully as possible to serve as predictive disease models. Missing human cardiac models that allow the development of therapies and predict responses in patients are the biggest bottlenecks towards much-needed treatments in cardiovascular disease. Our mission is to address this key problem affecting more patients than any other disease on the planet.
The branch of our lab at the Medical University of Vienna is in close collaboration with the cardiac branch of the lab at the Institute of Molecular Biotecnology (IMBA) at the Vienna Bio Center (https://www.oeaw.ac.at/imba/research/sasha-mendjan).
