Research Focus
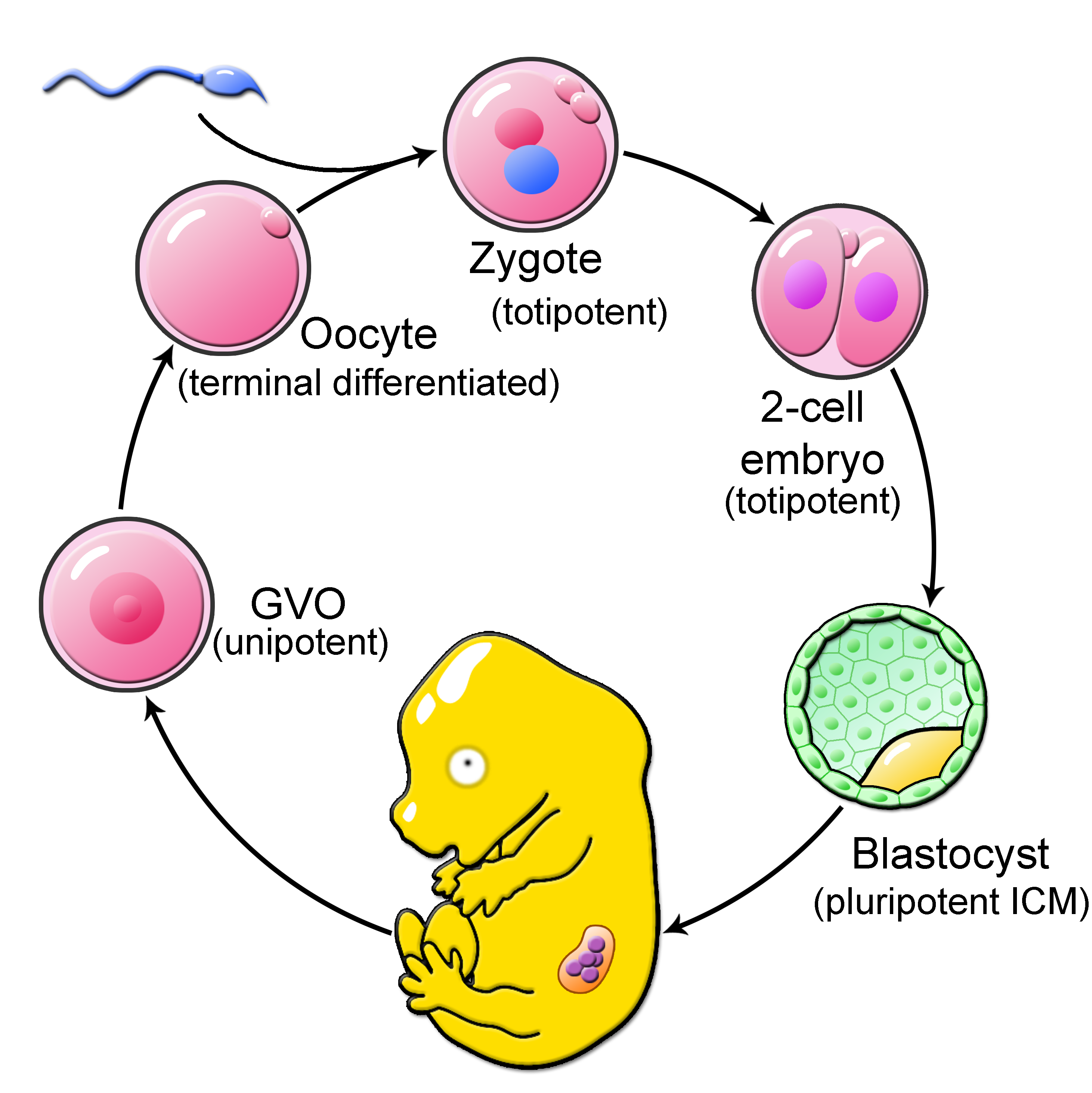
In early mammalian development, a little miracle is happening with every start of a new life. Here, shortly after fertilization, mammalian embryogenesis is characterized by dramatic epigenetic remodeling of the oocyte and sperm chromatin. This epigenetic reprogramming of the highly specialized egg and sperm epigenomes gives rise to the toti- and pluripotent blastomeres of preimplantation embryos, which are capable of generating all cell types needed to form a new life.
Most intriguingly, the genomes of early embryos undergo genome-wide DNA methylation (5-methyl-cytosine, 5mC) changes. Recent work suggests that DNA methylation reprogramming is an essential mechanism in early embryogenesis and the establishment of toti- and pluripotency. Knockout and Knockdown embryos for DNA methylation modifying enzymes have been shown with various developmental phenotypes in early and late stages of development. Moreover, pluripotent embryonic stem cells with abnormal DNA methylation are characterized by a loss of plasticity. How remodeling of DNA methylation by epigenetic modifiers is contributing to cellular potency in the early embryo and stem cell biology and how environmental cues are impacting this embryo intrinsic mechanism is poorly understood.
Main Objectives
Our group aims to elucidate the embryo intrinsic epigenetic reprogramming that drives early preimplantation development and the establishment of cellular potency. By analyzing epigenomic changes and probing the impact of specific epigenetic marks during early preimplantation development using novel genomic and epigenomic editing methods, we seek to understand the influence of epigenetic reprogramming on the establishment of toti-/pluripotency in vivo.
Content of Research
We use mouse embryos and embryonic stem cells in combination with novel genomic and epigenomic editing methods to investigate the impact of epigenetic changes and inter-/transgenerational inheritance of epimutations on development and cellular potency.
